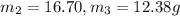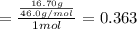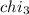Chemistry, 25.09.2019 00:20 latdoz0952
Aliquid mixture contains water (h2o, mw = 18.0), ethanol (c2h5oh, mw = 46.0) and methanol (ch3oh, mw = 32.0). using two different analytical techniques to analyze the mixture, it was determined that the water mole fraction was 0.250 while the water mass fraction was 0.134. determine the mole fraction ethanol (c2h5oh) and the mole fraction methanol (ch3oh) in the solution. report the values to the correct number of significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
Aliquid mixture contains water (h2o, mw = 18.0), ethanol (c2h5oh, mw = 46.0) and methanol (ch3oh, mw...
Questions

History, 11.05.2021 21:50


Biology, 11.05.2021 21:50

English, 11.05.2021 21:50


Mathematics, 11.05.2021 21:50

Chemistry, 11.05.2021 21:50



Mathematics, 11.05.2021 21:50






Mathematics, 11.05.2021 21:50

Mathematics, 11.05.2021 21:50



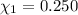
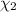
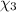
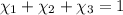
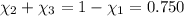
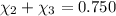
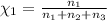
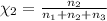
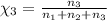
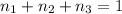
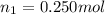
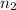
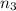
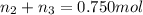
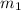
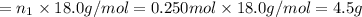
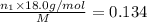
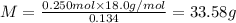
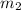
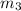
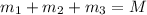
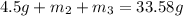
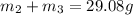 ..[1]
..[1]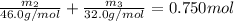
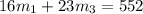 ..[2]
..[2]