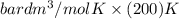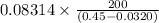Chemistry, 25.09.2019 01:00 nene3210204
Calculate the pressure exerted by ar for a molar volume 0.45 l at 200 k using the van der waals equation of state. the van der waals parameters a and b for ar are 1.355 bar dm mol-2 and 0.0320 dm3mol? , respectively. write your answer (unit: bar) with 2 decimals, as 12.23. do not add unit to your answer.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1
You know the right answer?
Calculate the pressure exerted by ar for a molar volume 0.45 l at 200 k using the van der waals equa...
Questions

Physics, 14.12.2021 04:00



Mathematics, 14.12.2021 04:00


Mathematics, 14.12.2021 04:00


Mathematics, 14.12.2021 04:00

SAT, 14.12.2021 04:00

Chemistry, 14.12.2021 04:00

Mathematics, 14.12.2021 04:00

Mathematics, 14.12.2021 04:00



Social Studies, 14.12.2021 04:00


Mathematics, 14.12.2021 04:00


English, 14.12.2021 04:10

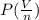 = RT
= RT 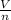 = v; which is called molar volume
= v; which is called molar volume = RT
= RT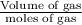
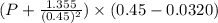 = 0.08314
= 0.08314