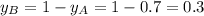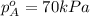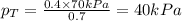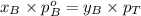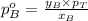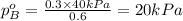Components a and b form ideal solution. at 350 k, a liquid mixture containing 40% (mole) a is in equilibrium with a vapour containing 70% (mole) a. if the vapour pressure of a at 350 k is 70 kpa, what is the vapour pressure of b? (b) 20 kpa (d) 12 kpa (а) 25 kpa (c) 40 kpa

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Which answer tells the reason the earth’s climate is getting warmer? too many animals are becoming extinct. large glaciers are melting in antarctica. the earth is moving closer to the sun. driving cars gives off gases that trap heat in the atmosphere.
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3

Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1
You know the right answer?
Components a and b form ideal solution. at 350 k, a liquid mixture containing 40% (mole) a is in equ...
Questions

Mathematics, 30.07.2019 03:00

Mathematics, 30.07.2019 03:00

Mathematics, 30.07.2019 03:00


Social Studies, 30.07.2019 03:00

History, 30.07.2019 03:00

English, 30.07.2019 03:00





Biology, 30.07.2019 03:00

Biology, 30.07.2019 03:00

Social Studies, 30.07.2019 03:00

Mathematics, 30.07.2019 03:00

Social Studies, 30.07.2019 03:00

Biology, 30.07.2019 03:00


English, 30.07.2019 03:00

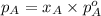 .............(1)
.............(1)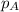 = partial vapor pressure of A
= partial vapor pressure of A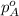 = vapor pressure of pure substance A
= vapor pressure of pure substance A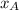 = mole fraction of A
= mole fraction of A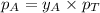 .............(2)
.............(2)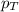 = total pressure of the mixture
= total pressure of the mixture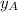 = mole fraction of A
= mole fraction of A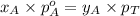
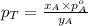 ............(3)
............(3)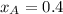 and
and 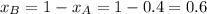
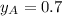 and
and