
Chemistry, 25.09.2019 01:10 cocacola8268
Radioactive decay can be described by the following equation in a = in ao- kt where ao is the original amount of the substance, a is the amount of the substance remaining after time t, and k is a constant that is characteristic of the substance. minutes for the radioactive isotope chromium-56, k is 1.17 x 10 if the original amount of chromium-56 in a sample is 34.5 mg, how much time is needed for the amount of chromium- 56 that remains to fall to 20.3 mg?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 07:30
Free answer. the treaty of versailles ended world war i, but some of the terms of the treaty contributed to the beginning of world war ii. which was one of the terms of the treaty? the answer would be "germany was forces to pay reparations to the allied countries.". i hope this .
Answers: 1

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3
You know the right answer?
Radioactive decay can be described by the following equation in a = in ao- kt where ao is the origin...
Questions








Mathematics, 17.01.2020 11:31

Mathematics, 17.01.2020 11:31


History, 17.01.2020 11:31

Mathematics, 17.01.2020 11:31

History, 17.01.2020 11:31

Computers and Technology, 17.01.2020 11:31

Mathematics, 17.01.2020 11:31

Mathematics, 17.01.2020 11:31


Mathematics, 17.01.2020 11:31

Mathematics, 17.01.2020 11:31

Mathematics, 17.01.2020 11:31

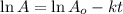
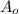 = initial mass of Cr-56 isotope = 34.5 mg
= initial mass of Cr-56 isotope = 34.5 mg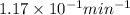
![\ln (20.3)=\ln (34.5)-[(1.17\times 10^{-1}min^{-1})\times t]}\\\\t=\frac{\ln(34.5)-\ln(20.3)}{1.17\times 10^{-1}}=4.53min](/tpl/images/0259/8505/00b1f.png)


