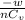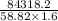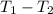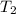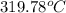Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 08:00
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 2

Chemistry, 23.06.2019 10:00
Two moles of potassium chloride and three moles of oxygen are produced from the decomposition of two moles of potassium chlorate, kcos3(s). write the balanced equation. how many moles of oxygen are produced from 12 moles of potassium chlorate
Answers: 1

Chemistry, 23.06.2019 13:30
These traits describe either a chemical or a nuclear reaction. which statements describe a nuclear reaction? check all that apply. involves the loss, gain, or sharing of electrons may involve a change in total mass involve relatively low energy changes occur outside the nucleus involve very high-energy changes involve changes in nuclides when decay occurs
Answers: 1
You know the right answer?
Consider 1 kg of nh3 that was compressed from 2.5 bar and 30°c to 5.0 bar in a well-insulated compre...
Questions

Mathematics, 31.01.2020 00:54






Physics, 31.01.2020 00:55

Health, 31.01.2020 00:55

French, 31.01.2020 00:55

Mathematics, 31.01.2020 00:55


History, 31.01.2020 00:55

Chemistry, 31.01.2020 00:55


Biology, 31.01.2020 00:55

Mathematics, 31.01.2020 00:55

World Languages, 31.01.2020 00:55

Mathematics, 31.01.2020 00:55


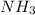 = 17 g/mol
= 17 g/mol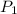 = 2.5 bar =
= 2.5 bar = 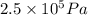 (as 1 bar =
(as 1 bar = 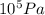 )
)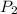 = 5 bar =
= 5 bar = 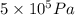
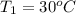 = 30 + 273 = 303 K
= 30 + 273 = 303 K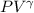 = constant = k
= constant = k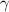 = 1.33 =
= 1.33 = 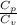
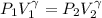
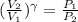
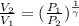
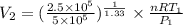 (as PV = nRT)
(as PV = nRT)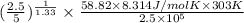
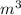
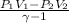
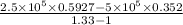
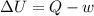
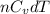 = -w
= -w