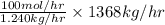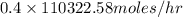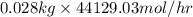Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1


Chemistry, 22.06.2019 21:50
28. which is not a reason that water is used to store spent fuel rods from nuclear power plants? water increases the speed of the chain reaction in the fuel rods. water protects nuclear power plant workers from the high temperature and radiation of the fuel rods. water acts as a radiation shield to reduce the radiation levels. water cools the spent rods. salts action
Answers: 1
You know the right answer?
Pure nitrogen (n2) and pure hydrogen (h2) are fed to a mixer. the product stream has 40.0% mole nitr...
Questions

Law, 26.01.2021 20:30







Mathematics, 26.01.2021 20:30



Mathematics, 26.01.2021 20:30


History, 26.01.2021 20:30

Computers and Technology, 26.01.2021 20:30


History, 26.01.2021 20:30


French, 26.01.2021 20:30


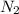 in feed = 40 mole%
in feed = 40 mole%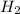 in feed = (100 - 40)% = 60%
in feed = (100 - 40)% = 60%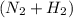 in feed stream.
in feed stream.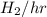 (2 g/mol of
(2 g/mol of 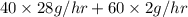
 (as 1 kg = 1000 g)
(as 1 kg = 1000 g)