Chemistry, 25.09.2019 02:00 AriesDaWolf
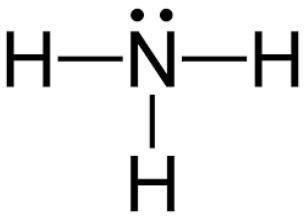
Which of the following explains the vsepr geometry of an ammonia molecule?
it is tetrahedral because there are four bonded pairs around nitrogen.
it is trigonal pyramidal because there are four bonded pairs around nitrogen.
it is tetrahedral because there are three bonded pairs and one lone pair around nitrogen.
it is trigonal pyramidal because there are three bonded pairs and one lone pair around nitrogen.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Subduction zones occur on earth where dense oceanic crust dives under more buoyant continental crust. these boundaries are characterized by a deep ocean trench next to a high continental mountain range, large numbers of earthquakes and volcanoes. all of this is further evidence for the a) big bang theory b) origin of the species eliminate c theory of plate tectonics d theory of natural selection 4 sedimentary rocks found high in the himalayen mountain
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3
You know the right answer?
Which of the following explains the vsepr geometry of an ammonia molecule?
it is tetrahedral...
it is tetrahedral...
Questions

Biology, 24.09.2019 04:30

Geography, 24.09.2019 04:30


History, 24.09.2019 04:30

History, 24.09.2019 04:30



Spanish, 24.09.2019 04:30


Mathematics, 24.09.2019 04:30

Health, 24.09.2019 04:30



Computers and Technology, 24.09.2019 04:30

English, 24.09.2019 04:30

Social Studies, 24.09.2019 04:30

Physics, 24.09.2019 04:30

Mathematics, 24.09.2019 04:30

Mathematics, 24.09.2019 04:30