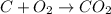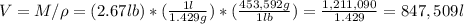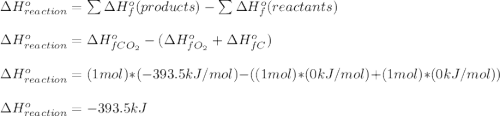Chemistry, 25.09.2019 01:30 kayleighanne3462
Every year people die from carbon monoxide poisoning because they bring a charcoal fire into their tent, house, or enclosed area. when carbon burns, it can produce either carbon dioxide, co2(g), or carbon monoxide gas, co(g).
a) if you start with a system containing 1 pound of charcoal briquettes (assuming pure carbon), how many grams of oxygen is needed to turn all of the carbon into the safe carbon dioxide?
b) what volume of air is required (in l)?
c) how much heat is given off as a result of the combustion to co2(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

You know the right answer?
Every year people die from carbon monoxide poisoning because they bring a charcoal fire into their t...
Questions


History, 28.06.2019 00:00


History, 28.06.2019 00:00

Mathematics, 28.06.2019 00:00

Mathematics, 28.06.2019 00:00

Biology, 28.06.2019 00:00


Spanish, 28.06.2019 00:00


Mathematics, 28.06.2019 00:00


History, 28.06.2019 00:00





Biology, 28.06.2019 00:00

Social Studies, 28.06.2019 00:00

Mathematics, 28.06.2019 00:00