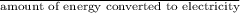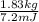Calculate the amount of co2 (in kg) released when 1 kg coal is burnt. assume that carbon content of the coal is 50% by mass. 4. calculate the co2 production in kg/mj if a coal fired power plant (efficiency - 30%) is used to produce electricity. assume energy density of coal - 24 mj/kg and assume the coal is

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 18:10
Measurements that have similar values are: a. usually accurate b. sometimes accurate c. always accurate d. never accurate
Answers: 1

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
Calculate the amount of co2 (in kg) released when 1 kg coal is burnt. assume that carbon content of...
Questions

World Languages, 20.07.2021 08:10


Social Studies, 20.07.2021 08:10



Physics, 20.07.2021 08:10

History, 20.07.2021 08:10

Mathematics, 20.07.2021 08:10

Mathematics, 20.07.2021 08:10

Physics, 20.07.2021 08:10

Physics, 20.07.2021 08:10


Mathematics, 20.07.2021 08:20

Spanish, 20.07.2021 08:20

Mathematics, 20.07.2021 08:20


Mathematics, 20.07.2021 08:20

Geography, 20.07.2021 08:20


Mathematics, 20.07.2021 08:20

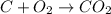
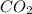 = 44 kg/kmol
= 44 kg/kmol
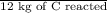
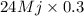 = 7.2 Mj
= 7.2 Mj