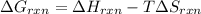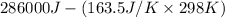Chemistry, 25.09.2019 02:20 destinyhammons12345
Determine the electrical work required to produce one mole of hydrogen in the electrolysis of liquid water at 298°k and 1 atm. the chemical reaction is h2001) h2(g) + 0.502(g) data (at 298°k and 1 atm): ah = 286 kj for this reaction, suzo = 70 jk, sh2 = 131 jik, and soz = 205 j/ºk.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2
You know the right answer?
Determine the electrical work required to produce one mole of hydrogen in the electrolysis of liquid...
Questions




English, 24.06.2020 09:01

Biology, 24.06.2020 09:01

Health, 24.06.2020 09:01

Mathematics, 24.06.2020 09:01


Mathematics, 24.06.2020 09:01



Mathematics, 24.06.2020 09:01


Mathematics, 24.06.2020 09:01


Mathematics, 24.06.2020 09:01


Mathematics, 24.06.2020 09:01


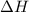 = 286 kJ =
= 286 kJ = 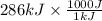
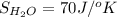 ,
, 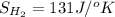
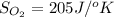
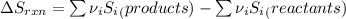
![[(\frac{1}{2} \times S_{O_{2}}) - (1 \times S_{H_{2}})] - [1 \times S_{H_{2}O}]](/tpl/images/0260/0439/5a90c.png)
![[(\frac{1}{2} \times 205) + (1 \times 131)] - [(1 \times 70)]](/tpl/images/0260/0439/31e9d.png)