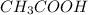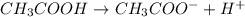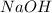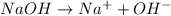Which statement best describes the conductivity of weak electrolytes, such as weak acids and bases?
a) weak electrolytes do not dissolve in water and can not conduct electricity.
b) weak electrolytes dissolve and partially dissociate in water providing charged ions to conduct electricity.
c) weak electrolytes dissolve and completely dissociate in water providing charged ions to conduct electricity.
d) weak electrolytes dissolve and does not dissociate in water providing no charged ions to conduct electricity.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1


Chemistry, 23.06.2019 05:00
What is dhmo? hint: you find it everywhere something is wet..
Answers: 1

Chemistry, 23.06.2019 06:00
Is the flow of energy during vaporizing more like the flow during melting or during freezing
Answers: 1
You know the right answer?
Which statement best describes the conductivity of weak electrolytes, such as weak acids and bases?...
Questions



Mathematics, 15.02.2021 19:00

Chemistry, 15.02.2021 19:00

Mathematics, 15.02.2021 19:00

Mathematics, 15.02.2021 19:00


Mathematics, 15.02.2021 19:00

Mathematics, 15.02.2021 19:00

Mathematics, 15.02.2021 19:00




Mathematics, 15.02.2021 19:00



English, 15.02.2021 19:00


English, 15.02.2021 19:00

Mathematics, 15.02.2021 19:00