
Chemistry, 26.09.2019 16:30 costel8532
An insulated thermos contains 150 g of water at 87.3 ˚c. you put in a 10.2 g ice cube at 0.00 ˚c to form a system of ice + original water. the specific heat of liquid water is 4190 j/kg•k; and the heat of fusion of water is 333 kj/kg. what is the net entropy change of the system from then until the system reaches the final (equilibrium) temperature?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Smog is the term used to describe the combination of fog and smoke
Answers: 1

Chemistry, 22.06.2019 00:00
1) this is the structure in the cell nucleus that houses a cell's genetic information
Answers: 3

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3

Chemistry, 22.06.2019 05:00
If the density of water is 1.0 g/cm3, which of these materials would float in water, based on their densities? check all that apply. aluminum cork iron lead wax
Answers: 1
You know the right answer?
An insulated thermos contains 150 g of water at 87.3 ˚c. you put in a 10.2 g ice cube at 0.00 ˚c to...
Questions


Social Studies, 26.07.2019 07:30

Chemistry, 26.07.2019 07:30

Biology, 26.07.2019 07:30






Arts, 26.07.2019 07:30

Arts, 26.07.2019 07:30

Arts, 26.07.2019 07:30

Arts, 26.07.2019 07:30

Arts, 26.07.2019 07:30

Arts, 26.07.2019 07:30

Arts, 26.07.2019 07:30

Social Studies, 26.07.2019 07:30

Computers and Technology, 26.07.2019 07:30

Computers and Technology, 26.07.2019 07:30

Arts, 26.07.2019 07:30

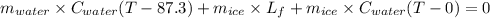
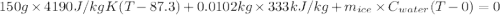
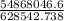
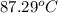
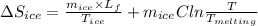
![\frac{0.0102 [\frac{333000}{273.15} + 4190 \times ln (\frac{360.44}{273.15})]](/tpl/images/0265/1324/75975.png)
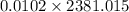
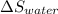 ) =
) = 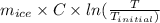
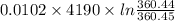
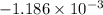 J/K
J/K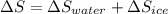
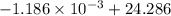 J/K
J/K

