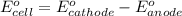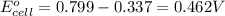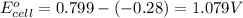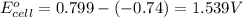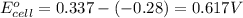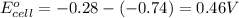Chemistry, 26.09.2019 16:30 savage5447
The standard reduction potentials of the following half-reactions are given in appendix e in the textbook:
ag+(aq)+e−→ag(s)= .799
cu2+(aq)+2e−→cu(s)= .337
ni2+(aq)+2e−→ni(s)= -.28
cr3+(aq)+3e−→cr(s). = -.74
1. determine which combination of these half-cell reactions leads to the cell reaction with the largest positive cell emf.
1st and 2nd,
1st and 3rd,
1st and 4th,
2nd and 3rd,
3rd and 4th.
it isn't the first or last one because i have gotten it wrong twice.
2. calculate the value of this emf.
3. then determine which combination is the smallest and calculate the emf.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What problem would a person have if the nucleic acid in one of his or her cells were damaged?
Answers: 2

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1
You know the right answer?
The standard reduction potentials of the following half-reactions are given in appendix e in the tex...
Questions



Biology, 28.08.2019 21:00



English, 28.08.2019 21:00




Mathematics, 28.08.2019 21:00




Mathematics, 28.08.2019 21:00


History, 28.08.2019 21:00




Social Studies, 28.08.2019 21:00

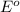 potential will always get reduced and will undergo reduction reaction.
potential will always get reduced and will undergo reduction reaction.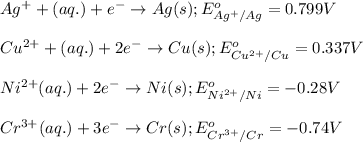
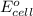 of the reaction, we use the equation:
of the reaction, we use the equation: