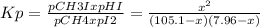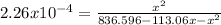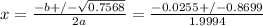Chemistry, 26.09.2019 17:30 Demondevilg
Methane, ch4, reacts with i2 according to the reaction ch4(g)+i2(g)⇌ch3i(g)+hi(g)
at 630 k, kp for this reaction is 2.26×10−4. a reaction was set up at 630 k with initial partial pressures of methane of 105.1 torr and of 7.96 torr for i2.
calculate the pressure, in torr, of ch4.
express your answer to four significant figures and include the appropriate units.
calculate the pressure, in torr, of i2.
express your answer to three significant figures and include the appropriate units.
calculate the pressure, in torr, of ch3i.
express your answer to three significant figures and include the appropriate units.
calculate the pressure, in torr, of hi.
express your answer to three significant figures and include the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1
You know the right answer?
Methane, ch4, reacts with i2 according to the reaction ch4(g)+i2(g)⇌ch3i(g)+hi(g)
at 630 k, kp...
at 630 k, kp...
Questions



History, 20.01.2020 09:31



Biology, 20.01.2020 09:31

Mathematics, 20.01.2020 09:31


Biology, 20.01.2020 09:31


Geography, 20.01.2020 09:31


History, 20.01.2020 09:31

Mathematics, 20.01.2020 09:31




Mathematics, 20.01.2020 09:31

Mathematics, 20.01.2020 09:31

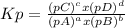 , where p is the partial pressure in the equilibrium. By the reaction given:
, where p is the partial pressure in the equilibrium. By the reaction given: