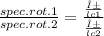An aqueous solution containing 10 g of an optically pure compound was diluted to 500 ml with water and was found to have a specific rotation of −129°. if this solution were mixed with 500 ml of a solution containing 7 g of a racemic mixture of the compound, what would the specific rotation of the resulting mixture of the compound? what would be its optical purity?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3
You know the right answer?
An aqueous solution containing 10 g of an optically pure compound was diluted to 500 ml with water a...
Questions


















Mathematics, 07.03.2020 00:41


History, 07.03.2020 00:41