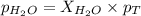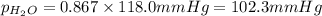Chemistry, 26.09.2019 19:00 Tyrant4life
What is the equilibrium partial pressure of water vapor above a mixture of 62.9 g h2o and 33.2 g hoch2ch2oh at 55 °c. the partial pressure of pure water at 55.0 °c is 118.0 mm hg. assume ideal behavior for the solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 10:30
Earth's axis of rotation is tilted at an angle of 23.5 degrees. what is one change you would see on earth if its axis was not tilted?
Answers: 3

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
What is the equilibrium partial pressure of water vapor above a mixture of 62.9 g h2o and 33.2 g hoc...
Questions

Mathematics, 01.12.2021 22:40


Mathematics, 01.12.2021 22:40




English, 01.12.2021 22:40


Computers and Technology, 01.12.2021 22:40

History, 01.12.2021 22:40

Mathematics, 01.12.2021 22:40

Social Studies, 01.12.2021 22:40



Biology, 01.12.2021 22:40



Biology, 01.12.2021 22:40

Mathematics, 01.12.2021 22:40

Physics, 01.12.2021 22:40

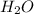 is 102.3 mmHg.
is 102.3 mmHg.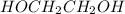 = 33.2 g
= 33.2 g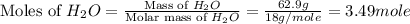
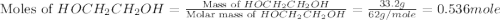
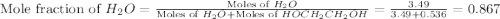
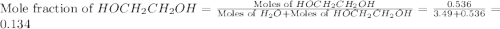
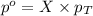
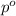 = partial pressure of water vapor
= partial pressure of water vapor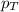 = total pressure of gas
= total pressure of gas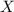 = mole fraction of water vapor
= mole fraction of water vapor