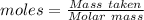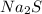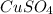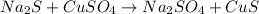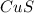What is the percent yield of cus for the following reaction given that you start with 15.5 g of na2s and 12.1 g cuso4? the actually amount of cus produced was 3.05 g. reaction: na2s + cuso4 → na2so4 + cus (a) 16.1% (b) 42.1% (c) 18.93% (d) 7.25% (e) not enough information

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

You know the right answer?
What is the percent yield of cus for the following reaction given that you start with 15.5 g of na2s...
Questions

Mathematics, 12.04.2021 21:30







Chemistry, 12.04.2021 21:30


Mathematics, 12.04.2021 21:30



English, 12.04.2021 21:30






Mathematics, 12.04.2021 21:30

English, 12.04.2021 21:30