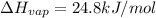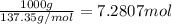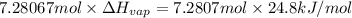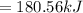Chemistry, 26.09.2019 19:30 Kekkdkskdkdk
Freon-11, ccl3f has been commonly used in air conditioners. it has a molar mass of 137.35 g/mol and its enthalpy of vaporization is 24.8 kj/mol at its normal boiling point of 24c. ideally how much energy in the form of heat is removed from a room by an air conditioner that evaporates 1.00 kg of freon-11?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 22.06.2019 10:00
Select all of the methods through which a drug can enter your body. injection swallowing inhalation absorption
Answers: 2

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
Freon-11, ccl3f has been commonly used in air conditioners. it has a molar mass of 137.35 g/mol and...
Questions


Mathematics, 26.03.2020 02:57

Mathematics, 26.03.2020 02:57




Physics, 26.03.2020 02:57

Mathematics, 26.03.2020 02:57


Chemistry, 26.03.2020 02:57


Mathematics, 26.03.2020 02:57

Social Studies, 26.03.2020 02:57


Spanish, 26.03.2020 02:57




Mathematics, 26.03.2020 02:58

Mathematics, 26.03.2020 02:58