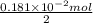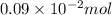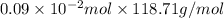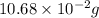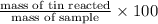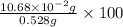Chemistry, 26.09.2019 22:20 dramaqueenactr2040
Find the percent by mass of tin in the original sample, assuming that it contains no other reducing agents. a sample of impure tin of mass 0.528 g is dissolved in strong acid to give a solution of sn2+. the solution is then titrated with a 0.0448 m solution of no3−, which is reduced to no(g). the equivalence point is reached upon the addition of 4.03×10−2 l of the no3− solution.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Find the percent by mass of tin in the original sample, assuming that it contains no other reducing...
Questions




English, 16.10.2020 07:01

History, 16.10.2020 07:01

History, 16.10.2020 07:01


Social Studies, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01

Biology, 16.10.2020 07:01

History, 16.10.2020 07:01




Mathematics, 16.10.2020 07:01

Mathematics, 16.10.2020 07:01

English, 16.10.2020 07:01


Mathematics, 16.10.2020 07:01

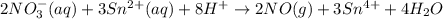
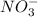 consumed will be calculated as follows.
consumed will be calculated as follows.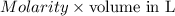
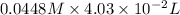
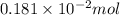
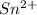 .
.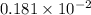 moles of
moles of