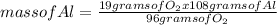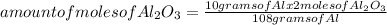Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 23.06.2019 01:00
Aman applies a force of 500n to push a truck 100m down the street how much does he do?
Answers: 1

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1
You know the right answer?
How many moles of aluminum oxide are produced according to the reaction below given that you start w...
Questions


Mathematics, 28.10.2019 00:43

History, 28.10.2019 00:43

Physics, 28.10.2019 00:43




Chemistry, 28.10.2019 00:43

Physics, 28.10.2019 00:43


Mathematics, 28.10.2019 00:43


Mathematics, 28.10.2019 00:43

Biology, 28.10.2019 00:43

Mathematics, 28.10.2019 00:43




History, 28.10.2019 00:43