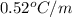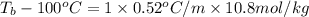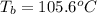Chemistry, 27.09.2019 00:30 Seaqueen3103
How many liters of the antifreeze ethylene glycol [ch2(oh)ch2(oh)] would you add to a car radiator containing 6.50 l of water if the coldest winter temperature in your area is –20ºc? calculate the boiling point of this water-ethylene glycol mixture. (te density of ethylene glycol is 1.11 g/ml.) kf = 1.86 ºc /m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3


Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2
You know the right answer?
How many liters of the antifreeze ethylene glycol [ch2(oh)ch2(oh)] would you add to a car radiator c...
Questions

Biology, 11.02.2021 03:00


Computers and Technology, 11.02.2021 03:00

English, 11.02.2021 03:00



English, 11.02.2021 03:00

Mathematics, 11.02.2021 03:00


Mathematics, 11.02.2021 03:00


Mathematics, 11.02.2021 03:00


Advanced Placement (AP), 11.02.2021 03:00



Mathematics, 11.02.2021 03:00

History, 11.02.2021 03:00


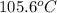
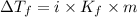
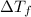 = change in freezing point =
= change in freezing point = 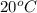
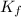 = freezing point constant =
= freezing point constant = 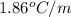
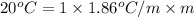
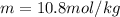
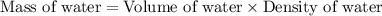
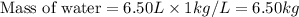
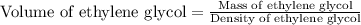
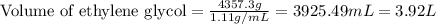
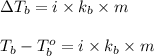
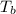 = boiling point of solution = ?
= boiling point of solution = ?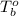 = boiling point of pure water =
= boiling point of pure water = 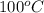
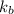 = boiling point constant =
= boiling point constant =