
Chemistry, 27.09.2019 01:10 maybeemmamay
In a particular experiment, 2.50-g samples of each reagent are reacted. the theoretical yield of lithium nitride is g. molar mass of li is 6.94 g/mol. molar mass of n2 is 28.02 g/mol. molar mass of li3n is 34.83 g/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 23.06.2019 00:30
What is calcium oxide+diphosphorus pentoxide--> calcium phosphate balanced
Answers: 1

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
You know the right answer?
In a particular experiment, 2.50-g samples of each reagent are reacted. the theoretical yield of lit...
Questions


Mathematics, 31.03.2020 17:59

Social Studies, 31.03.2020 17:59



History, 31.03.2020 17:59


Mathematics, 31.03.2020 17:59

History, 31.03.2020 17:59

Mathematics, 31.03.2020 17:59




Mathematics, 31.03.2020 17:59



Mathematics, 31.03.2020 17:59



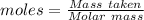
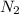
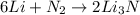
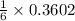 mole of
mole of 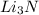
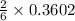 mole of
mole of 


