

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1


Chemistry, 22.06.2019 13:30
An animal cell loses the ability to convert energy stored in food to energy that the cell can use. which of the cell's organelles has stopped working? a.the mitochondria b.the nucleus c.the vacuoles d.the endoplasmic reticulum
Answers: 1

Chemistry, 22.06.2019 15:30
Count the number of each type of atom in the equation below, and then balance the equation. write in the numbers of atoms and coefficients. add a 1 if there should be no coefficient. cs2(l) + o2(g) → co2(g) + so2(g) c [ ] s [ ] o > c [ ] s [ ] o [ ] cs2(l) + [ ] o2(g) > [ ] co2(g) + [ ] so2(g)
Answers: 3
You know the right answer?
Calculate the amount of co2 produced when 1.00 mole of propane (c3h8) is burned in the presence of 1...
Questions

Biology, 08.10.2019 04:00


Mathematics, 08.10.2019 04:00


Geography, 08.10.2019 04:00


Mathematics, 08.10.2019 04:00




Social Studies, 08.10.2019 04:00

History, 08.10.2019 04:00

Computers and Technology, 08.10.2019 04:00







History, 08.10.2019 04:00

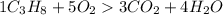
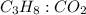 is 1: 3
is 1: 3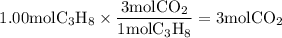
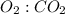 is 5: 3
is 5: 3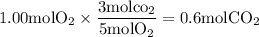
 formed is 0.600mol (Answer)
formed is 0.600mol (Answer)


