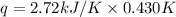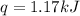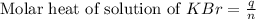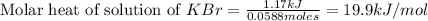Chemistry, 27.09.2019 02:30 bracefacer42
When a 7.00 g7.00 g sample of kbrkbr is dissolved in water in a calorimeter that has a total heat capacity of 2.72 kj⋅k−1,2.72 kj⋅k−1, the temperature decreases by 0.430 k.0.430 k. calculate the molar heat of solution of kbr.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1
You know the right answer?
When a 7.00 g7.00 g sample of kbrkbr is dissolved in water in a calorimeter that has a total heat ca...
Questions





English, 30.10.2020 23:30

Social Studies, 30.10.2020 23:30



Chemistry, 30.10.2020 23:30

Mathematics, 30.10.2020 23:30

Spanish, 30.10.2020 23:30


Mathematics, 30.10.2020 23:30

Mathematics, 30.10.2020 23:30

Mathematics, 30.10.2020 23:30

Mathematics, 30.10.2020 23:30

History, 30.10.2020 23:30

Mathematics, 30.10.2020 23:30


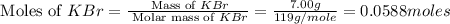
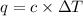
 = heat capacity =
= heat capacity = 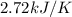
 = change in temperature = 0.430 K
= change in temperature = 0.430 K