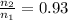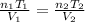Ahot-air balloon is filled with air to a volume of at 750. torr and 21°c. the air in the balloon is then heated to 58°c, causing the balloon to expand to a volume of . what is the ratio of the number of moles of air in the heated balloon to the original number of moles of air in the balloon? (hint: openings in the balloon allow air to flow in and out. thus the pressure in the balloon is always the same as that of the atmosphere.) ratio = 1

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
Ahot-air balloon is filled with air to a volume of at 750. torr and 21°c. the air in the balloon is...
Questions




Mathematics, 13.07.2020 20:01


Spanish, 13.07.2020 20:01

Mathematics, 13.07.2020 20:01

History, 13.07.2020 20:01








English, 13.07.2020 20:01

Mathematics, 13.07.2020 20:01

History, 13.07.2020 20:01