
Chemistry, 27.09.2019 03:30 Knownothing
Ethyl iodide (c2h5i) decomposes at a certain temperature in the gas phase as follows: c2h5i(g) → c2h4(g) + hi(g) from the following data determine the order of the reaction and the rate constant. time (min) [c2h5i] (m) 0.0 2.00 15.0 1.82 30.0 1.64 48.0 1.42 75.0 1.10

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 09:00
Water is a highly important natural resource. which of these would be the best method to conserve water? a) drinking bottled water b) monitoring the ph of rivers c) treating and re-using wastewater d) testing nitrate levels in groundwater
Answers: 1

Chemistry, 23.06.2019 14:00
If the molar mass of the compound is 96.69 g/mol, what is the molecular formula of the compound?
Answers: 1
You know the right answer?
Ethyl iodide (c2h5i) decomposes at a certain temperature in the gas phase as follows: c2h5i(g) → c2...
Questions


Mathematics, 25.05.2020 17:58

Mathematics, 25.05.2020 17:58

Physics, 25.05.2020 17:58


Social Studies, 25.05.2020 17:58


Mathematics, 25.05.2020 17:58

Mathematics, 25.05.2020 17:58


Physics, 25.05.2020 17:58

Mathematics, 25.05.2020 17:58

Mathematics, 25.05.2020 17:58

History, 25.05.2020 17:58

Mathematics, 25.05.2020 17:58

Social Studies, 25.05.2020 17:58

History, 25.05.2020 17:58

History, 25.05.2020 17:58

Mathematics, 25.05.2020 17:58

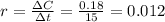
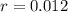 .
.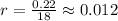
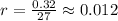
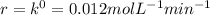 that is, the reaction is the zero order respect to C2H5I since it is not depending on concentration of C2H5I.
that is, the reaction is the zero order respect to C2H5I since it is not depending on concentration of C2H5I. 

