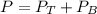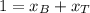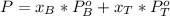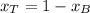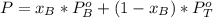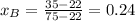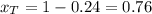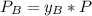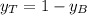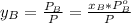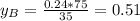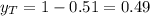Chemistry, 27.09.2019 04:10 chamarabrown9260
A) whatis the composition in mole fractions of a solution of benzene and toluene that has a vapor pressure of 35 torr at 20 °c? assume the mixture formsan ideal solution. the vapor pressure of benzene (c6h6) is 75 torr and the vapor pressure of toluene (c7h8)is 22 torr at 20 °c. b) what is the composition in mole fractions of the vapor above the solution in part a? how does this problem relate to the process of fractional distillation?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 09:30
Which ocean zone has the most abundant primary producer and why a) the abyssopelagic zone ,du to the absence of light and cold water temperatureb) the bathypelagic zone, due to the absence of light and cold water temperaturec) the mesopelagic zone ,due to uts high light availability and warm water temperature d) the epipelagic zone,due to its high light availability and warm water temperature
Answers: 3

Chemistry, 22.06.2019 16:00
How do dying stars contribute to the formation of planets
Answers: 1

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
A) whatis the composition in mole fractions of a solution of benzene and toluene that has a vapor pr...
Questions


English, 27.10.2020 23:30

Business, 27.10.2020 23:30

Chemistry, 27.10.2020 23:30

Mathematics, 27.10.2020 23:30




English, 27.10.2020 23:30





Mathematics, 27.10.2020 23:30

Mathematics, 27.10.2020 23:30

Mathematics, 27.10.2020 23:30

Chemistry, 27.10.2020 23:30




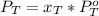
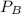 is partial pressure for benzene in the liquid
is partial pressure for benzene in the liquid 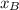 is benzene molar fraction in the liquid
is benzene molar fraction in the liquid 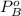 vapor pressure for pure benzene.
vapor pressure for pure benzene.