
Achemistry student needs 50.0 g of methyl acetate for an experiment. by consulting the crc handbook of chemistry and physics, the student discovers that the density of methyl acetate is 0.934 g. cm . calculate the volume of methyl acetate the student should pour out. round your answer to 3 significant digits. x s ?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
Achemistry student needs 50.0 g of methyl acetate for an experiment. by consulting the crc handbook...
Questions

Mathematics, 24.06.2019 15:00




History, 24.06.2019 15:00

Mathematics, 24.06.2019 15:00

Computers and Technology, 24.06.2019 15:00

Computers and Technology, 24.06.2019 15:00

Chemistry, 24.06.2019 15:00



History, 24.06.2019 15:00

Biology, 24.06.2019 15:00





Social Studies, 24.06.2019 15:00


Physics, 24.06.2019 15:00

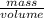
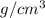 .
.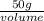
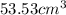
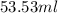 (as 1
(as 1 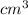 = 1 mL)
= 1 mL)

