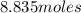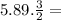Answers: 3


Another question on Chemistry




Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Given the balanced reaction equation below, how many moles of nitrogen are formed when 5.89 moles of...
Questions




Computers and Technology, 21.07.2019 23:00


Mathematics, 21.07.2019 23:00


Biology, 21.07.2019 23:00

Social Studies, 21.07.2019 23:00


Social Studies, 21.07.2019 23:00





Computers and Technology, 21.07.2019 23:00

Computers and Technology, 21.07.2019 23:00

History, 21.07.2019 23:00

Computers and Technology, 21.07.2019 23:00

Geography, 21.07.2019 23:00