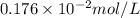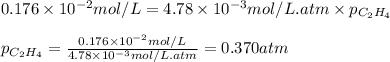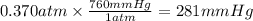Chemistry, 28.09.2019 01:30 MIAkwicc39
What partial pressure of c2h4 gas (in mm hg) is required to maintain a solubility of 4.92×10-2 g/l in water at 25 °c? kh for c2h4 at 25 °c is 4.78×10-3 mol/l·atm.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 17:30
Upon decomposition, one sample of magnesium fluoride produced 1.65 kg of magnesium and 2.56 kg of fluorine. a second sample produced 1.32 kg of magnesium. part a how much fluorine (in grams) did the second sample produce?
Answers: 2

Chemistry, 22.06.2019 23:30
Aweight lifter raises a 1600 n barbell to a height of 2.0 meters. how much work was done? w = fd a) 30 joules b) 3000 joules c) 320 joules d) 3200 joules
Answers: 2
You know the right answer?
What partial pressure of c2h4 gas (in mm hg) is required to maintain a solubility of 4.92×10-2 g/l i...
Questions

Mathematics, 27.02.2020 14:19


Mathematics, 27.02.2020 14:23

Mathematics, 27.02.2020 14:26


Mathematics, 27.02.2020 14:26

Computers and Technology, 27.02.2020 14:26

Mathematics, 27.02.2020 14:27

History, 27.02.2020 14:27

Mathematics, 27.02.2020 14:28

Mathematics, 27.02.2020 14:32

Mathematics, 27.02.2020 14:43


English, 27.02.2020 14:45


Mathematics, 27.02.2020 14:46

Mathematics, 27.02.2020 14:46

Mathematics, 27.02.2020 14:48

Mathematics, 27.02.2020 14:49

 is 281 mmHg
is 281 mmHg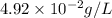
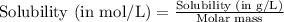
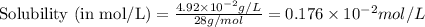
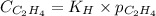
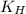 = Henry's constant =
= Henry's constant = 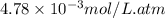
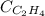 = molar solubility of ethene gas =
= molar solubility of ethene gas =