
Chemistry, 28.09.2019 02:10 Sylnatius6736
4. al2o3 (s) + 6hcl (aq) → 2alcl3 (aq) + 3h20(1) find the mass of alcl3 that is produced when 10.0 grams of al2o3 react with 10.0 g of hci moar mass alqo3 102 gm imol al2o3 = 0.098 moles molar mass of hcl = 36, 5gr/mol #of moles #6l =0,274 moles mole of al2 oz 6 mol of hc 01274 x2 = 0.0913 moles alc3=13,5 6 i mass alco3 = 12, 193gm. 5. how many grams of the excess reagent in question 4 are left over?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3


Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
4. al2o3 (s) + 6hcl (aq) → 2alcl3 (aq) + 3h20(1) find the mass of alcl3 that is produced when 10.0 g...
Questions


Medicine, 15.01.2021 08:30

Social Studies, 15.01.2021 08:30


Mathematics, 15.01.2021 08:30



Advanced Placement (AP), 15.01.2021 08:30


Chemistry, 15.01.2021 08:30



Mathematics, 15.01.2021 08:30

Mathematics, 15.01.2021 08:30


Mathematics, 15.01.2021 08:30



Mathematics, 15.01.2021 08:30

Mathematics, 15.01.2021 08:30

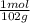 = 0,0980 moles
= 0,0980 moles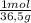 = 0,274 moles
= 0,274 moles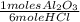 = 0,0457 moles of Al₂O₃
= 0,0457 moles of Al₂O₃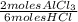 × 133
× 133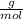 = 12,1 g of AlCl₃
= 12,1 g of AlCl₃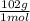 = 5,33 g of Al₂O₃
= 5,33 g of Al₂O₃ 

