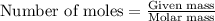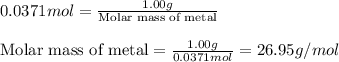Chemistry, 28.09.2019 02:10 sierranicole114
A1.00 g sample of a metal x (that is known to form x ions in solution) was added to 127.9 ml of 0.5000 m sulfuric acid. after all the metal had reacted, the remaining acid required 0.03340 l of 0.5000 m naoh solution for complete neutralization. calculate the molar mass of the metal and identify the element.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 17:20
Pegmatites are igneous rocks in which the individual minerals are very large. typically, the minerals are all light-colored quartz, feldspar and muscovite. if you were given a black and white photograph of a pegmatite in a quarry (where the rock has been blasted and broken), what physical properties could you use to identify those three minerals in this hypothetical photo? describe each mineral and the specific diagnostic properties. be specific.
Answers: 2

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2

Chemistry, 23.06.2019 07:30
Which of the following statements best explains why chemistry is testable a) it can measure data by experiments b) it cannot add new evidence c) it cannot be verified d) it is biased
Answers: 1
You know the right answer?
A1.00 g sample of a metal x (that is known to form x ions in solution) was added to 127.9 ml of 0.50...
Questions



Mathematics, 14.12.2020 20:10

Biology, 14.12.2020 20:10

Mathematics, 14.12.2020 20:10



Mathematics, 14.12.2020 20:10

Mathematics, 14.12.2020 20:10


Mathematics, 14.12.2020 20:10

Mathematics, 14.12.2020 20:10

Mathematics, 14.12.2020 20:10



Mathematics, 14.12.2020 20:10


Physics, 14.12.2020 20:10

English, 14.12.2020 20:10

Mathematics, 14.12.2020 20:10

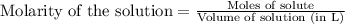 .....(1)
.....(1)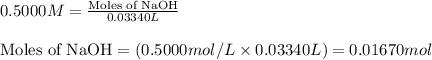

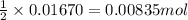 of sulfuric acid
of sulfuric acid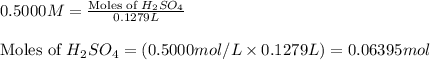
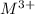 ion) and sulfuric acid follows:
ion) and sulfuric acid follows: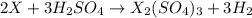
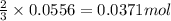 of metal
of metal