
Chemistry, 28.09.2019 02:30 ayoismeisjuam
Consider the gas reaction: h2 (g)2(g) 2hi (g) the equilibrium constant at 731 k is 50.3. equal amounts of all three gases (0.100 m) are introduced in a container, calculate the concentration of each gas after the system reaches equilibrium. express your results with the right number of significant figures.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 08:40
Write the formula for the following chemicals. 7. e. trinitrogen tetraoxide a calcium phosphate f. magnesium acetate b. potassium sulfide g nickel(iii) cyanide c carbon dioxide h. silver sulfate d. cobalt(ii) chloride
Answers: 1
You know the right answer?
Consider the gas reaction: h2 (g)2(g) 2hi (g) the equilibrium constant at 731 k is 50.3. equal amou...
Questions


Mathematics, 29.06.2021 03:50


Mathematics, 29.06.2021 03:50

Mathematics, 29.06.2021 03:50






Mathematics, 29.06.2021 04:00



Mathematics, 29.06.2021 04:00


Mathematics, 29.06.2021 04:00



Mathematics, 29.06.2021 04:00

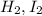 and
and 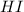 at equilibrium 0.033 M, 0.033 M and 0.234 M respectively.
at equilibrium 0.033 M, 0.033 M and 0.234 M respectively.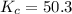
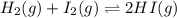
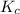 will be,
will be,![K=\frac{[HI]^2}{[H_2][I_2]}](/tpl/images/0269/8551/8a740.png)
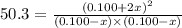
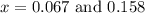
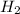 and
and 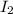 at equilibrium = (0.100-x) = 0.100 - 0.067 = 0.033 M
at equilibrium = (0.100-x) = 0.100 - 0.067 = 0.033 M

