
Chemistry, 28.09.2019 02:30 erikabermudez55
Consider an atom with 6 protons, 6 neutrons, and 6 electrons. state the number of protons, neutrons, and electrons that would occur in a different isotope of the same element, and explain what changed by relating the change to the definition of an isotope.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
If you want to create an electrical current, which situation would produce a solution capable of this
Answers: 3

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
Consider an atom with 6 protons, 6 neutrons, and 6 electrons. state the number of protons, neutrons,...
Questions


Mathematics, 04.03.2021 18:50

History, 04.03.2021 18:50


Mathematics, 04.03.2021 18:50

English, 04.03.2021 18:50



Health, 04.03.2021 18:50


Mathematics, 04.03.2021 18:50

Mathematics, 04.03.2021 18:50

Mathematics, 04.03.2021 18:50

Physics, 04.03.2021 18:50


Mathematics, 04.03.2021 18:50

Mathematics, 04.03.2021 18:50



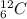 and
and 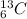 are isotopes.
are isotopes.

