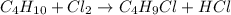Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 06:30
1.17 mol hcl and 2.5 mol naoh react according to the equation hcl + naoh -> nacl + h2o . if the limiting reactant is hcl, determine the amount of excess reactant that remains. answer in units of mol.
Answers: 1

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2

Chemistry, 23.06.2019 13:30
Which of the following is true regarding chemical and nuclear reactions?
Answers: 1

Chemistry, 23.06.2019 14:00
Tinererining 01: 57: 44 which statement correcte describes the actual veld and the theoretical yield of a reaction? textual vec is calculated to the reactant amounts but the theoretical yeld must be measured for each instance of a the actual vec is calculated from the amount of the limiting reactant and the theoretical yield is calculated from the 发公主 the actual weld depends on the reaction centers, but the theoretical yield and only with reactant amounts the actual vele represents the maximum weld possible and the theoretical yield assumes perfect reaction conditions save and ext e அட
Answers: 2
You know the right answer?
Given the equation for a reaction: c4h10 + cl2 → c4h9cl + hcl which type of reaction is represented...
Questions

History, 05.05.2020 16:04





History, 05.05.2020 16:04




Mathematics, 05.05.2020 16:04

Mathematics, 05.05.2020 16:04





Mathematics, 05.05.2020 16:04