
Chemistry, 28.09.2019 03:10 erikacastro2421
Which of the following is spontaneous at satp? o h2(g)—2h(9) o hg((9) o n2(g)+2o2(g)+9 kj—n204(9) o co2(s)-co2(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 23.06.2019 00:30
An ice cube with a volume of 45.0ml and a density of 0.9000g/cm3 floats in a liquid with a density of 1.36g/ml. what volume of the cube is submerged in the liquid
Answers: 3
You know the right answer?
Which of the following is spontaneous at satp? o h2(g)—2h(9) o hg((9) o n2(g)+2o2(g)+9 kj—n204(9) o...
Questions

Mathematics, 05.12.2019 06:31

Biology, 05.12.2019 06:31




Chemistry, 05.12.2019 06:31


Mathematics, 05.12.2019 06:31



Mathematics, 05.12.2019 06:31

Mathematics, 05.12.2019 06:31

Mathematics, 05.12.2019 06:31



Arts, 05.12.2019 06:31


Mathematics, 05.12.2019 06:31

Social Studies, 05.12.2019 06:31

English, 05.12.2019 06:31

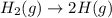
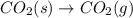 here number of moles remain the same. Hence, the reaction is not spontaneous.
here number of moles remain the same. Hence, the reaction is not spontaneous.

