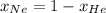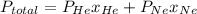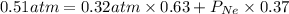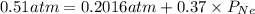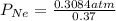Chemistry, 28.09.2019 03:30 dylanclark4965
Agas mixture has a total pressure of 0.51 atm and consists of he and ne. if the partial pressure of he in the mixture is 0.32 atm, what is the partial pressure of the ne in the mixture? enter your answer in the provided box. atm

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
The electron configurations of two different atoms are shown below. each yellow electron has a charge of 1−, and the net charge of each nucleus is shown. these atoms will combine with bond. a. an ionic b. a positive c. a negative d. a covalent plzzz mee with !
Answers: 1

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2


Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Agas mixture has a total pressure of 0.51 atm and consists of he and ne. if the partial pressure of...
Questions


Arts, 31.12.2019 22:31

Arts, 31.12.2019 22:31

Mathematics, 31.12.2019 22:31







English, 31.12.2019 22:31

History, 31.12.2019 22:31



Mathematics, 31.12.2019 22:31

Mathematics, 31.12.2019 22:31

History, 31.12.2019 22:31

Mathematics, 31.12.2019 22:31

Mathematics, 31.12.2019 22:31

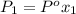
 = partial pressure of gas 1
= partial pressure of gas 1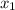 = mole fraction of gas 1
= mole fraction of gas 1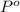 = total pressure of the gases
= total pressure of the gases 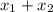 = 1
= 1 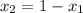
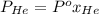
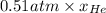
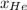 = 0.63
= 0.63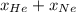 = 1
= 1