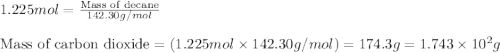Chemistry, 28.09.2019 03:30 kyramks421
Various members of a class of compounds, alkenes, react with hydrogen to produce a corresponding alkane. termed hydrogenation, this type of reaction is used to produce products such as margarine. a typical hydrogenation reaction is c10h20() + h2(g) → c10h22(5) decene decane how much decane can be produced in a reaction of excess decene with 2.45 g hydrogen? give your answer in scientific notation. o *10 g decane

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
Various members of a class of compounds, alkenes, react with hydrogen to produce a corresponding alk...
Questions

Mathematics, 12.08.2020 05:01






Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01


English, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01








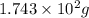
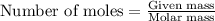 ......(1)
......(1)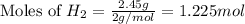
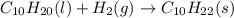
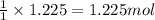 of decane
of decane