
Chemistry, 28.09.2019 04:10 StephenCurry34
Acertain liquid x has a normal freezing point of 7.60 °c and a freezing point depression constant k= 6.90 °c-kg-mol. calculate the freezing point of a solution made of 7.57g of sodium chloride (nacl) dissolved in 350. g of x round your answer to 3 significant digits. lºc x 5

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
Acertain liquid x has a normal freezing point of 7.60 °c and a freezing point depression constant k=...
Questions


Mathematics, 25.11.2020 14:20



Mathematics, 25.11.2020 14:20

Mathematics, 25.11.2020 14:20

Mathematics, 25.11.2020 14:20













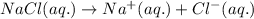
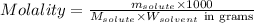
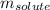 = Given mass of solute (NaCl) = 7.57 g
= Given mass of solute (NaCl) = 7.57 g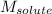 = Molar mass of solute (NaCl) = 58.44 g/mol
= Molar mass of solute (NaCl) = 58.44 g/mol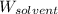 = Mass of solvent (liquid X) = 350.0 g
= Mass of solvent (liquid X) = 350.0 g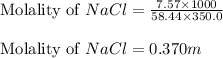
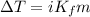
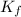 = molal freezing point depression constant = 6.90°C/m
= molal freezing point depression constant = 6.90°C/m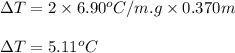
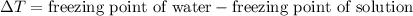
 = 5.11 °C
= 5.11 °C


