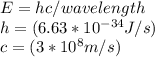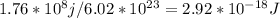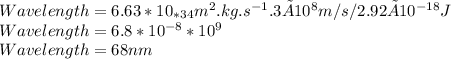Chemistry, 30.09.2019 18:20 mpgleboski
Determine the longest wavelength of light required to remove an electron from a sample of potassium metal, if the binding energy for an electron in k is 1,76x10*3 kj/mol.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 3

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2
You know the right answer?
Determine the longest wavelength of light required to remove an electron from a sample of potassium...
Questions

Biology, 23.11.2020 21:40


Biology, 23.11.2020 21:40

English, 23.11.2020 21:40

Mathematics, 23.11.2020 21:40

Mathematics, 23.11.2020 21:40


Physics, 23.11.2020 21:40

History, 23.11.2020 21:40


Mathematics, 23.11.2020 21:40






Mathematics, 23.11.2020 21:40