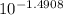Chemistry, 30.09.2019 21:00 geunagillis1
Abuffered solution containing dissolved aniline, c6h5nh2, and aniline hydrochloride, c6h5nh3cl, has a ph of 5.75.
a) determine the concentration of c6h5nh3+ in the solution if the concentration of c6h5nh2 is 0.245 m. the pkb of aniline is 9.13.
[c6h5nh3+]= m
b) calculate the change in ph of the solution, \delta ph, if 0.360g naoh is added to the buffer for a final volume of 1.50l.
\deltaph=

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1



Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
Abuffered solution containing dissolved aniline, c6h5nh2, and aniline hydrochloride, c6h5nh3cl, has...
Questions





Mathematics, 09.10.2021 02:50



Mathematics, 09.10.2021 02:50

Mathematics, 09.10.2021 02:50

Biology, 09.10.2021 02:50



Mathematics, 09.10.2021 02:50



Mathematics, 09.10.2021 02:50



Chemistry, 09.10.2021 02:50

![pH = pKa + log\frac{[A^-]}{[HA]}](/tpl/images/0277/5492/19c19.png)