
Chemistry, 30.09.2019 20:30 poweradampower
2n2h4(l) + n2o4(l) → 3n2(g) + 4h2o(g) [balanced] how many moles of n2h4 is required to produce 28.3 g of n2? assume that all reactants react completely. molar mass of n2h4 = 32.06 g/mol molar mass of n2o4 = 92.02 g/mol molar mass of n2 = 28.02 g/mol molar mass of h2o = 18.02 g/mol group of answer choices

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3


Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
2n2h4(l) + n2o4(l) → 3n2(g) + 4h2o(g) [balanced] how many moles of n2h4 is required to produce 28.3...
Questions

Social Studies, 10.10.2019 08:10


Advanced Placement (AP), 10.10.2019 08:10

Mathematics, 10.10.2019 08:10

Mathematics, 10.10.2019 08:10


Biology, 10.10.2019 08:10







Geography, 10.10.2019 08:10





Mathematics, 10.10.2019 08:10

Chemistry, 10.10.2019 08:10

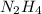
 of particles.
of particles.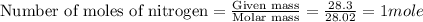
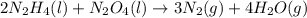
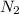 is produced by 2 moles of
is produced by 2 moles of 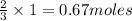 of
of 

