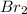Chemistry, 30.09.2019 21:10 brendacauani12345
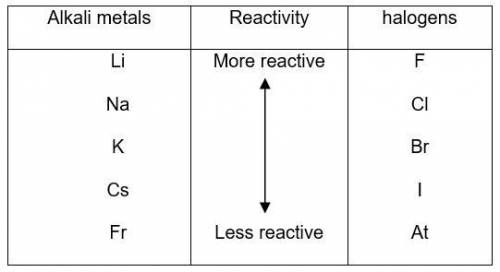
The elements in alkali metal and halogen groups of the periodic table are the most reactive since they only need to gain or lose one electron to become stable by filling their valence orbital. what happens to reactivity, moving down the column of a group?
a) reactivity stays the same because they are in the same group.
b) reactivity increases because the valence level is further from the nucleus of the atom.
c) reactivity decreases because the increased number of protons and electrons provides more stability.
d) reactivity stays the same because the number of electrons needed to fill the valence orbital remains the same.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3
You know the right answer?
The elements in alkali metal and halogen groups of the periodic table are the most reactive since th...
Questions


History, 13.07.2019 10:30

English, 13.07.2019 10:30

Business, 13.07.2019 10:30

Business, 13.07.2019 10:30



Mathematics, 13.07.2019 10:30



History, 13.07.2019 10:30


Mathematics, 13.07.2019 10:30


Mathematics, 13.07.2019 10:30



Mathematics, 13.07.2019 10:30


Advanced Placement (AP), 13.07.2019 10:30

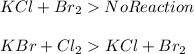
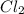 is more reactive than
is more reactive than