Background info:
the standard enthalpy of formation (δh∘f) is the enthalpy change that occurs...

Chemistry, 19.08.2019 10:30 terryonsavage543
Background info:
the standard enthalpy of formation (δh∘f) is the enthalpy change that occurs when exactly1 mol of a compound is formed from its constituent elements under standard conditions. the standard conditions are 1 atm pressure, a temperature of 25 ∘c , and all the species present at a concentration of 1 m . a "standard enthalpies of formation table" containing δh∘f values might look something like this: substanceδh∘fh(g)218 kj/molh2(g)0 kj/molba(s)0 kj/molba2+(aq)−538.4 kj/molc(g)71 kj/molc(s)0 kj/moln(g)473 kj/molo2(g)0 kj/molo(g)249 kj/mols2(g)129 kj/mol
question:
what is the balanced chemical equation for the reaction used to calculate δh∘f of baco3(s)? if fractional coefficients are required, enter them as a fraction (i. e. 1/3). indicate the physical states using the abbreviation (s), (l), or (g) for solid, liquid, or gas, respectively. use (aq) for aqueous solution.
express answer as a chemical equation. explain for me !

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 19:30
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2

You know the right answer?
Questions


Social Studies, 30.07.2019 08:30


History, 30.07.2019 08:30

Mathematics, 30.07.2019 08:30


English, 30.07.2019 08:30

History, 30.07.2019 08:30




Mathematics, 30.07.2019 08:30




Social Studies, 30.07.2019 08:30

Health, 30.07.2019 08:30



Mathematics, 30.07.2019 08:30

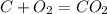
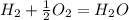
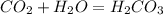
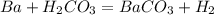
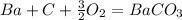 , using hydrogen as a catalyst.
, using hydrogen as a catalyst.

