
Chemistry, 01.10.2019 04:10 bettybales1986
A26.08 g mixture of zinc and sodium is reacted with a stoichiometric amount of sulfuric acid. the reaction mixture is then reacted with 132 ml of 3.80 m barium chloride to produce the maximum possible amount of barium sulfate. determine the percent sodium by mass in the original mixture.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Pls! plant cells and animal cells were observed under a microscope. the characteristics of two cells are listed below. cell p: does not capture sunlight cell q: has cytoplasm but no chloroplast which statement about the two cells is correct? cell q also has a cell wall. cell q also has large vacuole. cell p also has a large vacuole. cell p also has a cell membrane.
Answers: 1

Chemistry, 22.06.2019 17:00
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
A26.08 g mixture of zinc and sodium is reacted with a stoichiometric amount of sulfuric acid. the re...
Questions


Social Studies, 15.04.2021 21:40

Mathematics, 15.04.2021 21:40


History, 15.04.2021 21:40


Mathematics, 15.04.2021 21:40

Mathematics, 15.04.2021 21:50

Mathematics, 15.04.2021 21:50

Computers and Technology, 15.04.2021 21:50


Social Studies, 15.04.2021 21:50

Mathematics, 15.04.2021 21:50

Mathematics, 15.04.2021 21:50


Social Studies, 15.04.2021 21:50

Biology, 15.04.2021 21:50

Mathematics, 15.04.2021 21:50

Mathematics, 15.04.2021 21:50

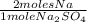 × 22,99 g/mole = 23,08 g of Na
× 22,99 g/mole = 23,08 g of Na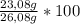 = 88,50%
= 88,50% 

