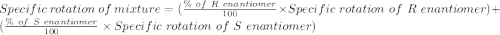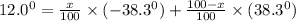Chemistry, 01.10.2019 04:10 mayahgrimes
An attempt at synthesizing a certain optically active compound resulted in a mixture of its enantiomers. the mixture had an observed specific rotation of 12.0°. if it is known that the specific rotation of the r enantiomer is –38.3°, determine the percentage of each isomer in the mixture.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
An attempt at synthesizing a certain optically active compound resulted in a mixture of its enantiom...
Questions




Arts, 25.07.2019 17:00




History, 25.07.2019 17:00


Geography, 25.07.2019 17:00





Social Studies, 25.07.2019 17:00


Chemistry, 25.07.2019 17:00

Health, 25.07.2019 17:00

Biology, 25.07.2019 17:00