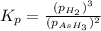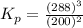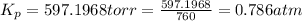Chemistry, 01.10.2019 04:20 gadgetady5699
The gas arsine (ash3) decomposes as follows: 2ash3(g) < > 2as(s) + 3h2(g) in an experiment pure ash3(g) was placed in an empty, rigid, sealed flask at a pressure of 392.0 torr. after 48 h the pressure in the flask was observed to be constant at 488.0 torr. a. calculate the equilibrium pressure of h2(g). b. calculate kp for this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Asample of radium-226 will decay 1/4 of its original amount after 3200years. what is the half-life of radium-226?
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2


You know the right answer?
The gas arsine (ash3) decomposes as follows: 2ash3(g) < > 2as(s) + 3h2(g) in an experiment p...
Questions

Health, 20.08.2021 08:10

Mathematics, 20.08.2021 08:10





Mathematics, 20.08.2021 08:10


Business, 20.08.2021 08:10

Chemistry, 20.08.2021 08:10

English, 20.08.2021 08:10



Mathematics, 20.08.2021 08:10

Advanced Placement (AP), 20.08.2021 08:10


English, 20.08.2021 08:10

Mathematics, 20.08.2021 08:10


 gas is, 288 torr
gas is, 288 torr for this reaction is, 0.786 atm
for this reaction is, 0.786 atm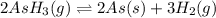
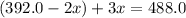
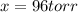
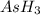 at equilibrium = (392.0-2x) = [392.0-2(96)] = 200 torr
at equilibrium = (392.0-2x) = [392.0-2(96)] = 200 torr