
You are in a submarine, the hallway in front of you is connected to two rooms. the hallway has a capacity of 300.0 l and is filled with 35.0 atm of helium gas. room a has a capacity of 200.0 l and holds 20.0 atm of nitrogen gas. room b has 40.0 atm of oxygen gas in 180.0 l. a. if you open both rooms to the hallway, what would be pressure of each gas in the combined space of the hallway and two rooms? b. what would be the mol fraction of oxygen in that gas? c. after mixing the gases, if you let gas escape until the total pressure in the hallway was 1.2 atm, what would be the final partial pressure of oxygen?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which is the layer underground where all empty spaces are filled with a combination of air and water ?
Answers: 1

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
You know the right answer?
You are in a submarine, the hallway in front of you is connected to two rooms. the hallway has a cap...
Questions






Mathematics, 20.09.2020 14:01

English, 20.09.2020 14:01

Mathematics, 20.09.2020 14:01


Mathematics, 20.09.2020 14:01



History, 20.09.2020 14:01

Mathematics, 20.09.2020 14:01




Mathematics, 20.09.2020 14:01


Spanish, 20.09.2020 14:01

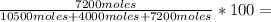 33,2% O₂
33,2% O₂

