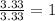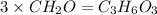Chemistry, 01.10.2019 17:30 MansellS5529
An elemental analysis is performed in an unknown compound. it is found to contain 40.0 % mass in carbon, 6.71% mass in hydrogen, and the remaining mass in oxygen. determine its empirical formula. the formula mass of the unknown is independently determined to be 90.08 g/mol, determine the unknown’s molecular formula

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:40
Can someone me with 6 to 10 plz this is for masteries test.
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
An elemental analysis is performed in an unknown compound. it is found to contain 40.0 % mass in car...
Questions

History, 30.10.2019 21:31







Mathematics, 30.10.2019 21:31

Mathematics, 30.10.2019 21:31


Mathematics, 30.10.2019 21:31

Mathematics, 30.10.2019 21:31

Mathematics, 30.10.2019 21:31




Biology, 30.10.2019 21:31


Mathematics, 30.10.2019 21:31

Chemistry, 30.10.2019 21:31