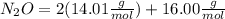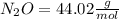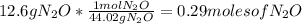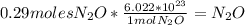Chemistry, 01.10.2019 17:30 arianawelsh123l
Dinitrogen monoxide or laughing gas (n2o) is used as a dental anesthetic and as an aerosol propellant. how many moles of n2o are present in 12.6 g of the compound? how many molecules of n2o are present in 12.6 g of the compound? [use molar masses: n, 14.01 g/mol, o, 16.00 g/mol]

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 21.06.2019 22:20
Which of the following statements is false regarding aromaticity? a. the compound must be cyclic b. the compound must be fully conjugated c. the compound must be planar d.the number of electrons in the pi system must satisfy the hückel 4n+2 rule e. the compound must have a neutral charge
Answers: 2

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1
You know the right answer?
Dinitrogen monoxide or laughing gas (n2o) is used as a dental anesthetic and as an aerosol propellan...
Questions

Mathematics, 17.12.2020 05:20

Mathematics, 17.12.2020 05:20







Chemistry, 17.12.2020 05:20

Geography, 17.12.2020 05:20

Health, 17.12.2020 05:20

Arts, 17.12.2020 05:20

Mathematics, 17.12.2020 05:20

Mathematics, 17.12.2020 05:20

Mathematics, 17.12.2020 05:20

Mathematics, 17.12.2020 05:20




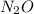 there are 0.29 moles of
there are 0.29 moles of 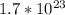 molecules of
molecules of